Researchers at Sweden-based Chalmers University of Technology have unveiled an innovative antibacterial surface coating based on this year’s Nobel Prize–winning material, metal-organic frameworks (MOF). The technology uses microscopic MOF spikes to physically puncture and kill bacteria. It offers a promising, antibiotic-free solution to the persistent problem of biofilms across healthcare and industry.
Biofilm-forming bacteria remain one of the most persistent challenges across healthcare, marine industries, and industrial infrastructure. Once bacteria colonize a surface, they rapidly multiply and shield themselves inside a protective biofilm. This slimy, resilient matrix makes them exceptionally hard to eradicate. From hospital-acquired infections to fouled ship hulls and corroded industrial piping, biofilms are an expensive and dangerous adversary.
Now, researchers at Chalmers University of Technology (Sweden) say they’ve identified an entirely new line of defense. One that doesn’t rely on antibiotics, biocides, or toxic metals. Their solution? A mechanical antibacterial surface made from this year’s Nobel Prize–winning material: metal-organic frameworks (MOFs).
Tiny MOF Spikes That Physically Kill Bacteria
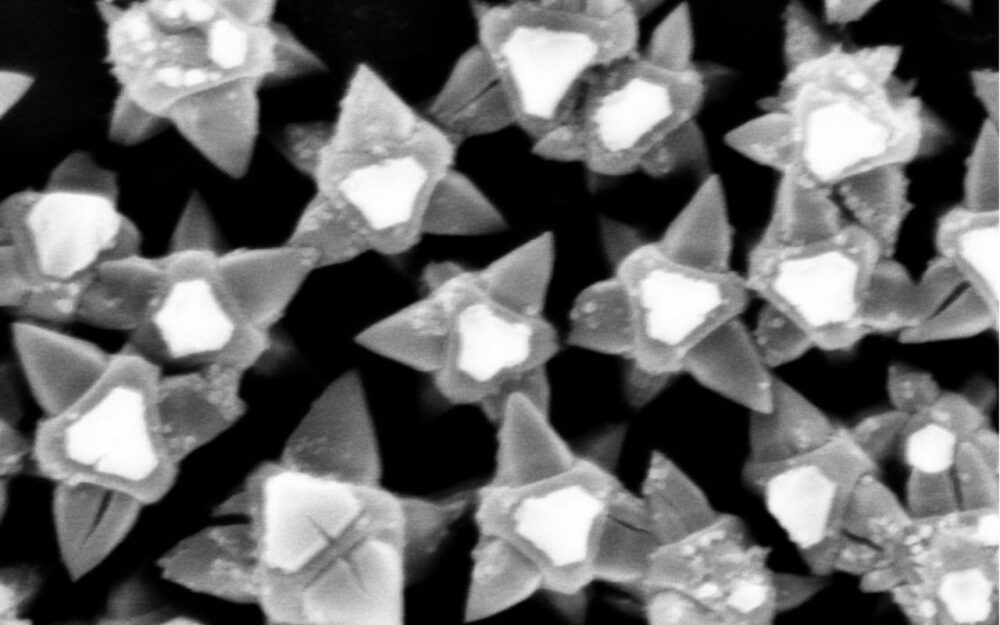
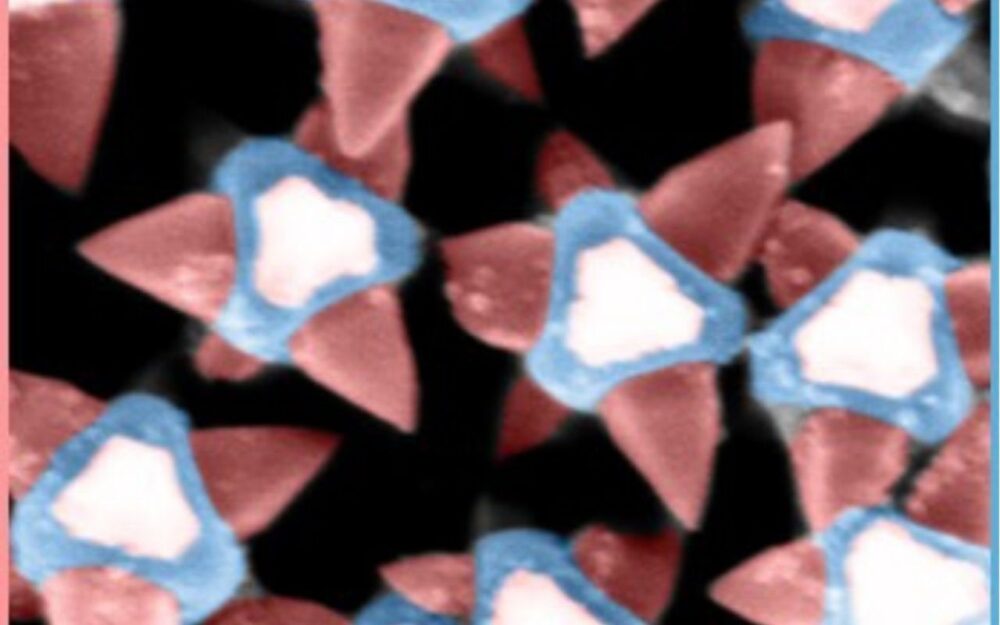
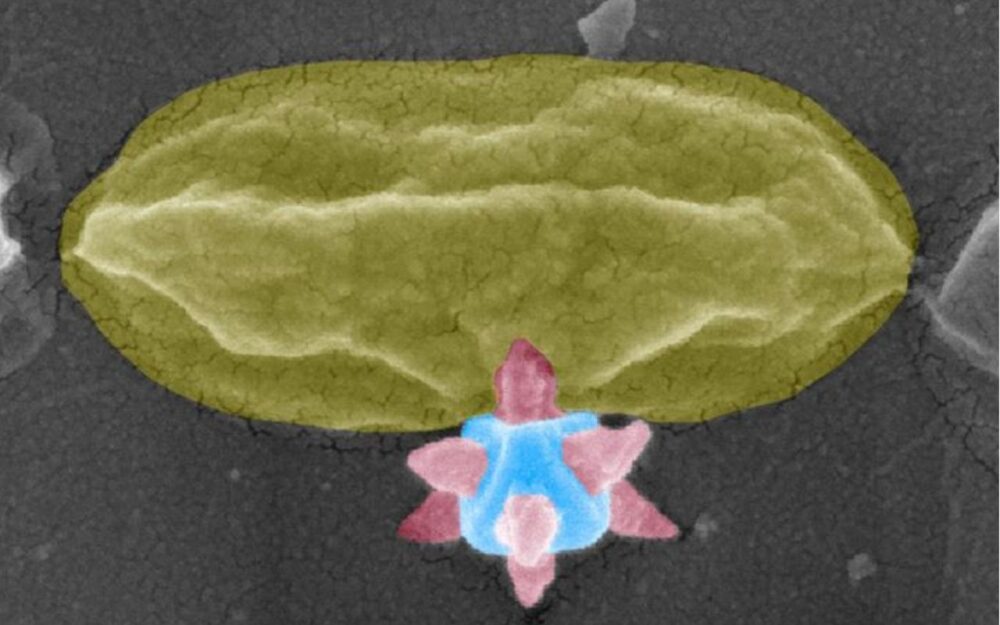
In a press release, the Chalmers team explained they have engineered a coating composed of MOF-based nanostructures that act as microscopic spikes. When bacteria come into contact with the surface, these nanotips puncture the bacterial membrane before the organism has a chance to attach and form a biofilm.
“It’s a completely new way of using such metal-organic frameworks,” explains Zhejian Cao, lead author and researcher in Materials Engineering at Chalmers.
Unlike previous MOF-based antimicrobial concepts—where the material releases toxic metal ions—this approach is purely mechanical. The coating functions as a physical bactericidal layer, eliminating concerns about antibiotic resistance or environmental contamination.
Why Spacing Matters: Too Close, and Bacteria Survive
One of the key engineering challenges was determining the optimal spacing between nanotips. If they’re too far apart, bacteria slip between them. Too close, and the pressure each tip applies is too small. This phenomenon is similar to lying on a bed of nails without injury.
By adjusting crystalline growth during fabrication, the researchers were able to fine-tune tip geometry and distribution to maximize bactericidal efficiency.
Temperature-Friendly Manufacturing for Sensitive Materials
Co-author and MOF specialist Lars Öhrström highlights another advantage: scalability. Unlike graphene-based bactericidal surfaces—which require high-temperature production—MOF coatings can be grown at significantly lower temperatures.
This means they can be applied to temperature-sensitive polymers commonly used in medical implants, a major market opportunity for manufacturers. Moreover, the organic components of MOFs can be synthesized from recycled plastics, supporting circular-economy production models.
Industrial Impact: Beyond Healthcare
While the press release emphasizes clinical applications—such as catheters, dental implants, and joint replacements—the implications extend far beyond hospitals:
In shipbuilding and marine operations, this technology could enable non-toxic antifouling surfaces that eliminate the need for harmful biocides.
Regarding the chemical and process industries, it could be used to protect piping and reactor surfaces from corrosion-inducing biofilms.
In water treatment applications, the approach may help create biofilm-free membranes and filtration systems.
In the food and beverage sector, it could lead to more hygienic equipment that requires less cleaning and experiences reduced downtime.
For manufacturers seeking to reduce operational costs, energy losses, and maintenance cycles, mechanical antibiofilm technologies could open the door to next-generation “self-defending” materials.

A Breakthrough Material with Wide Horizons
MOFs were awarded the 2025 Nobel Prize in Chemistry for their unique porous structures and wide applicability in carbon capture, catalysis, and gas storage. The Chalmers research, however, introduces a completely new function to their repertoire.
The study—Mechano-Bactericidal Surfaces Achieved by Epitaxial Growth of Metal-Organic Frameworks—was published in Advanced Science and funded by multiple Nordic and European research foundations.
This development marks a promising shift toward antibiotic-free, chemical-free, and energy-efficient antimicrobial surfaces. For industries where biofouling and biofilm formation lead to significant losses—from hospitals to shipyards—MOF nanotip coatings could represent a new class of protective materials ready for future integration into industrial design and manufacturing.
If scalable production advances as expected, MOF-based bactericidal coatings may soon become a competitive differentiator in markets seeking sustainability, safety, and long-term durability.
Read also








![Image [Best of 2025] Power Moves in the Energy World](/wp-content/uploads/sites/3/energy-320x213.jpg)