Advances in medical imaging, computational power, and numerical algorithms allow medical companies to simulate human environments. Researchers are now able to model the entire human body accurately, potentially transforming medical research and product development. The American Food and Drug Administration (FDA) has already authorized the market release of medical devices tested through digital simulation. We spoke with Christophe Bianchi, CTO at Ansys about the advantages of computer-based testing in the medical field.
Digital Evidence vs. Clinical Evidence
In the past, medical understanding evolved through two main approaches: In Vivo: Researchers conducted tests and experiments on living organisms and cadavers to understand how the human body works. In Vitro: Experiments were conducted in test tubes and petri dishes, leading to innovations in the pharmaceutical industry.
Now, a growing number of experiments are done on computers to complement and accelerate in vivo and in vitro approaches. This is called in-silico medicine and this new approach has the potential to revolutionize science and medicine. The concept is to simulate the interaction of medical products with the body without the need for real-body testing.
The American FDA has already authorized the market release of medical devices tested through digital simulation.
According to Christophe Bianchi, CTO at simulation software provider Ansys,
“In-silico addresses several concerns: development time, time to market, and market entry costs. It also allows testing of many more candidates and much more development. Additionally, if we can avoid sacrificing animals for testing because we have validated the use of digital models as sufficient, it is very good.”
Advantages of Computer-Based Testing for Rare Diseases
The primary advantage of in-silico medicine pertains to rare diseases. The validation process of a drug follows different phases. The initial phases involve analysis, toxicity, and risk assessment. Then, there are efficacy analyses. And then, there are analyses on very large populations or cohorts. When working on medications for extremely common diseases, there are no cohort issues.
“But when working on extremely rare diseases, it is sometimes nearly impossible to establish a cohort. So the drug cannot be validated because there are not enough patients to test it on. Hence in-silico testing.”
And we all witnessed it during COVID-19 when a vaccine swiftly entered the market. Digital simulation played a pivotal role as it facilitated the testing of numerous configurations.
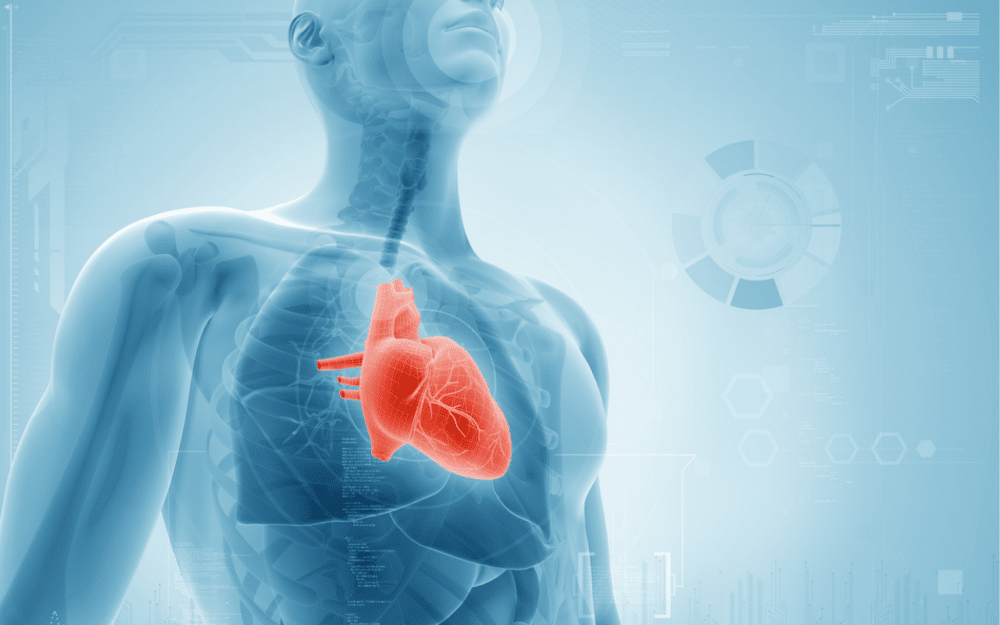
A Digital Twin of the Human Body
But what exactly is tested with simulation? Pharmacokinetics or pharmacodynamics can be tested—how quickly a molecule will act on the target area and its effectiveness. Toxicity can also be assessed, such as whether a medication a patient is taking poses a risk of causing a heart attack.
“Today, we can assess the toxicity of a molecule through simulation. By creating a digital twin of the heart from a database of MRI scans of hearts from a thousand patients, we can represent a civilian population accurately. Essentially, a digital heart functions like a computer program. So, this allows us to configure it to match various demographics, including age, weight, and gender.”
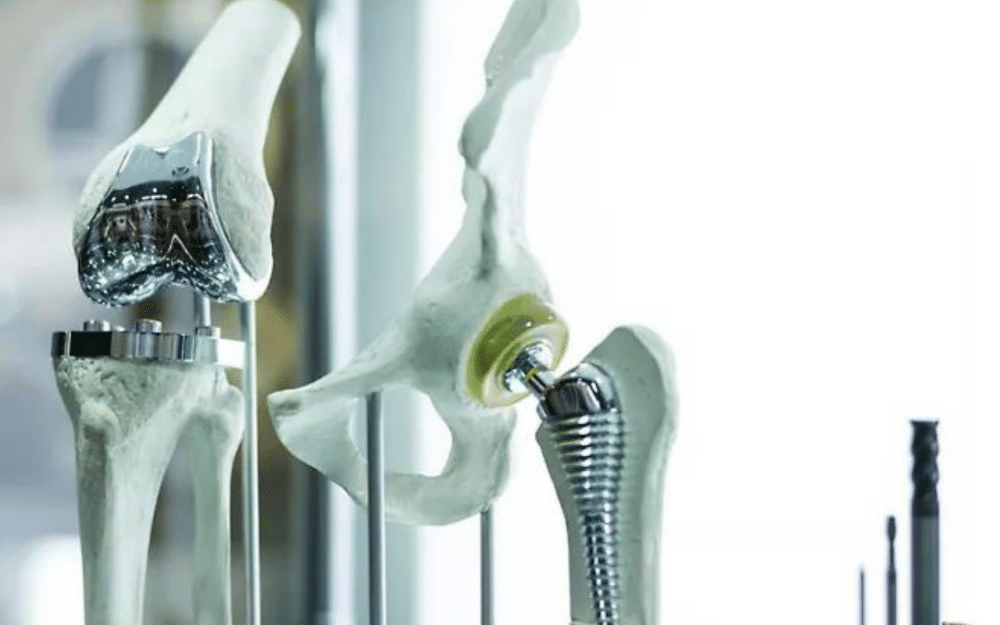
When evaluating a stent, for instance, to ensure proper deployment, testing occurs on the computer model rather than on patients. Similarly, when testing a new orthopedic screw for splint fixation, digital simulations using models of elbows, arms, or knees are also employed.
Furthermore, simulation enables the creation of significantly larger virtual cohorts, which is crucial in the case of rare diseases.
More Accurate Simulation
Unlike the digital twin of an engine or a factory, which is a CAD representation, a virtual organ is created using measurements taken in the laboratory. Therefore, simulation has the unexpected effect of being more precise than real testing.
“When conducting a clinical trial, testing a drug on a patient doesn’t yield very precise results due to the significant variability from one patient to another. Additionally, factors like diet, timing of ingestion, and unforeseen events further contribute to the lack of precision in real clinical trials compared to simulations. Therefore, simulations provide more qualitative information than actual clinical studies.”
Besides, analyzing data from a group of patients and extracting meaningful information is much easier to accomplish using numerical models than human models, Mr. Bianchi says:
“Human data is often imperfect and unreliable; for example, MRI images may have poor quality. This unreliability necessitates extremely large cohorts in clinical studies to draw definitive conclusions about a product’s efficacy and safety. Simulations offer precise measurements and analyses based on well-defined equations, eliminating the need for extensive human testing.”
Regulatory Challenges
In the USA, the Food and Drug Administration has already approved drugs and medical devices that have undergone digital simulation instead of being tested on patients before their market release.
“If we develop a new inhaler to deliver asthma treatment, we can simulate precisely what happens when you press the inhaler and how the molecules dissipate in the airways using models of different patients. Regarding a stent, to ensure it deploys correctly, we do it using computer modeling. Today, this has become almost standard.”
Generics are also another example of a drug that can be tested digitally, with no need for a clinical study.
If the consideration of in-silico digital evidence is something that is fully acknowledged at the FDA level, Europe is a bit further behind. Ansys is collaborating with European regulators to ensure that digital evidence is accepted on par with clinical evidence.