Will we soon be able to manufacture human organs like we produce tires or semiconductors? On the East Coast of the United States, some have the ambition to establish a new industry related to regenerative medicine within a burgeoning “ReGen Valley”, a medical counterpart to the renowned Silicon Valley on the West Coast. They intend to leverage the expertise of major industrial players such as Rockwell Automation to establish this innovative ecosystem. Earlier this month, DirectIndustry had the opportunity to visit ARMI, the Advanced Regenerative Manufacturing Institute located in Manchester, New Hampshire, just an hour north of Boston.
It is always challenging to get excited about medical projects with futuristic ambitions that claim to change the world by promising to heal humanity, especially after the Theranos scandal. In 2018, Elizabeth Holmes, an American former biotechnology entrepreneur, was convicted of fraud related to her blood-testing company, Theranos. She promised to revolutionize blood testing with technology that required only a few drops of blood. Despite millions of dollars in investments, the technology never worked. In January 2022, Holmes was found guilty of four counts of defrauding investors. In November 2022, she was sentenced to 11 years in prison.
Since this scandal, we journalists have looked at medical and biomedical initiatives with a more skeptical eye. Despite this, research continues, and many are seeking new ways to heal us. Will they succeed?
Manufacturing Replacement Human Organs: A New Industry
In any case, Dean Kamen, the Founder of FIRST Robotics (who notably developed the famous Segway), Director of Deka Research, and founder of the Advanced Regenerative Manufacturing Institute (ARMI), believes in it. This former veteran even aims to create a whole new industry – nothing less than the industry of producing replacement human organs for soldiers, veterans, and other patients.
Dean’s observation is clear: the United States spends 21% of its GDP on healthcare, primarily for chronic care issues such as diabetes, kidney problems, dialysis, and others. Chronic kidney diseases, for example, cost $114 billion a year according to the Department of Health & Human Services (HHS). The cost of diabetes was $307 billion in 2022. For Dean Kamen,
“That’s gonna be going up at an astounding rate. We can reverse that trend. Give people a better quality of life if we figure out how to replace the failed organs and do it at scale and do it with quality. Nobody wants these chronic treatments. They want cures. Once the healthcare system can make it practical to give you a new pancreas instead of wearing a pump for the rest of your life or a new kidney instead of being on dialysis three times a day for the rest of your life, the quality of life goes up, the cost goes down. If we build an industry that makes it practical to start supplying replacement organs, it will change the overall economy of this country and our export capability.”

Developing this future industry is also vital for the US. The genesis of the ARMI concept can be traced back to discussions held during the last Obama administration. At that time, Obama wanted to establish several industries aimed at safeguarding America’s leadership in core technologies including biotechnology.
As a result, ARMI was launched in 2017 as a member-driven and non-profit organization with the objective of jump-starting the large-scale production of synthetic human tissue.
And it can rely on the support of the U.S. Department of Defense, which granted $80 million, and, more surprisingly, on Rockwell Automation, the American leader in automation and one of ARMI’s founding board members.
Earlier this month, we rode to Manchester, New Hampshire, just an hour north of Boston, to visit ARMI and learn more about the project, including how to establish a new industry and its connection with Rockwell, a company more accustomed to equipping the manufacturing world. Dean led the tour in this giant 80,000-square-foot building, which aims to be the world’s first facility entirely dedicated to manufacturing and scaling replacement organs.
Automating the Production of Human Tissues
ARMI has a dual objective: to facilitate the manufacturing of human tissue for organs and to automate and democratize the industrial process to make it possible, for example, to install desktop units directly in clinics and medical offices. Eventually, spare parts for patients with serious injuries or diseases could include lungs, pancreas, livers, and even hearts.
Manufacturing human cells is something researchers have known how to do for a long time. The significant challenge is to do it at scale, not only in laboratories but in manufacturing facilities, and in a way that is cost-effective, explains Tom Bollenbach, Chief Technology Officer at ARMI, who holds a Ph.D. in biochemistry.
“There’s been a tremendous investment on the academic side into tissue engineering cell therapies. Despite that, since the 1970s when Eugene Bell published on the production of skin in his lab in 1976, there really have only been a handful of project products that have reached the market. That’s because manufacturing and scale-up have really been misinterpreted by scientists, rather than engineers.”
His team has developed a prototype of a tissue foundry as part of ARMI’s project, BioFabUSA, which features an automated production line for manufacturing cells. The tissue foundry aims to scale up production for GMP readiness, utilizing automation modules for unit operations and custom-engineered components integrated into a closed and automated process. This process must ensure critical quality attributes and adhere to scalable FDA-compliant manufacturing processes.
“The challenge here is that we’re using a human cell as a raw material and the human cells are as variable as the people in this room right now. Imagine producing a batch of cells or a tissue with your cells for you specifically. And the challenge goes up even another order of magnitude. So here we are trying to solve that challenge. ”
And the challenge is huge. The goal is to transplant organs using the patient’s own genetics to minimize or eliminate the risk of rejection issues. In order to do that, there is the need to create a tissue-manufacturing platform that is scalable (for commercial production), modular (to adapt to changing variables), automated (to control critical process parameters to ensure consistency), and closed (to avoid the need for expensive clean-room spaces).
For Tom,
“We aim to be enablers of mass production.”
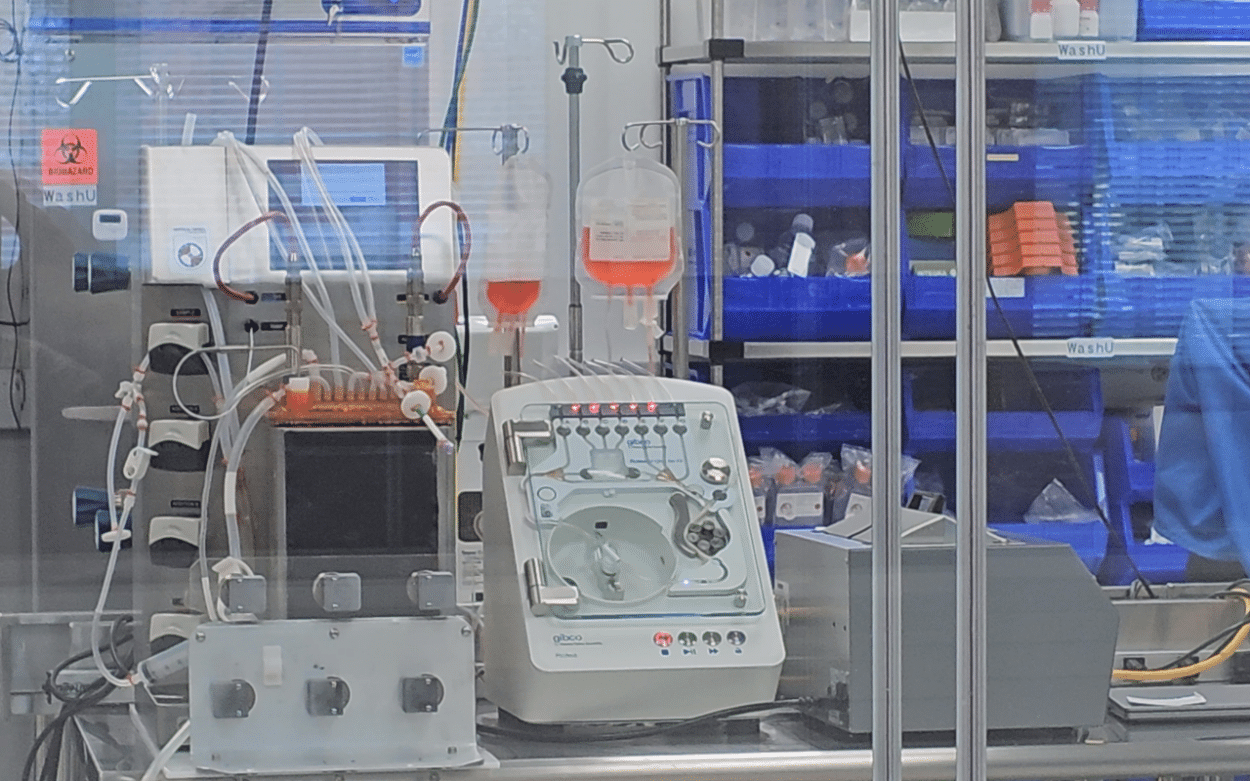
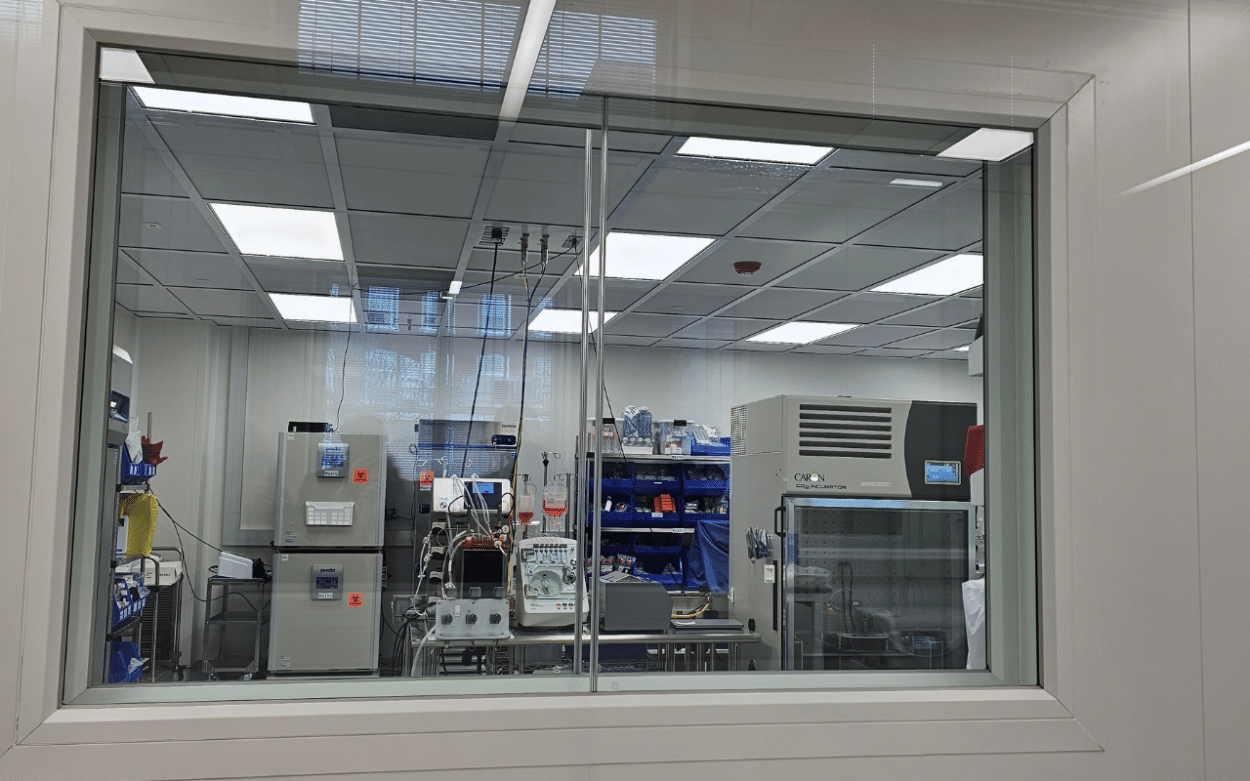
The Tissue Foundry Line
Tom and Dean took us on a tour of the tissue line. It involves several stations starting with the harvest of a blood sample or tissue biopsy, followed by cell isolation and amplification. The process involves combining cells with a scaffold material, incubating it to form tissue, and then packaging and shipping it. The process is 45 days long and leads to a product that is ready to implant.
The modular design allows the automation of major steps, such as cell expansion, harvest, wash, tissue assembly, and maturation. The scalable system can handle various cell types and produce different tissues, including ACLs, blood rotator cuffs, and skeletal muscle. Several sensors enable it to monitor and measure the various steps. The whole process is also monitored by a distributed control system (DCS).
A crucial consideration is how to establish quality control for a living entity, explained Tom:
“How do you even quality control a living thing? What do you even ask about that cell or that tissue that tells you it’s gonna be efficacious in the patient? Because at the end of the day, quality control is really asking the question, what do I measure that tells me that this thing is gonna work in the hands of the user and that’s universally true for any product.”
Dean added:
“It’s a very complicated thing to create an industry, not a product, not a company, but an industry. To get an industry up and running from nothing, we’re gonna need standards. We’re gonna need to get standardized ways to have the Food and Drug Administration accept quality systems and quality data that the scientific community doesn’t know how to do. And we need to build those systems.”
So, what criteria can be used to assess the efficacy of a cell or tissue in a patient? What technology can be mobilized to enhance quality control and automate the process to obtain a reliable and consistent product? For Dean, Rockwell Automation was one valuable partner.
“To get to that scale quickly, we need companies like Rockwell. They brought to the table what’s worked in every other industry and they can help make it work for us. We need all the expertise they use on a daily basis in highly developed well-defined regulated industries, the semiconductor industry, the automotive industry, and the aerospace. We need to create that substrate. We need this massive infrastructure to turn this into a high-volume business.”
The Role of Rockwell Automation’s Equipment
Rockwell has thus entered the board of ARMI. During the Automation Fair in Boston, to which DirectIndustry was invited, the CEO of Rockwell Automation, Blake Moret, said:
“The actual advanced regenerative medicine institute near New Hampshire shows what we’re actually doing in terms of regenerative medicine. And for me, that’s one of the clearest examples of how we’re making this real, taking the concept of tissue regeneration and doing it at scale with the knowledge and expertise that Rockwell has.”
The foundry therefore features a multitude of small hardware modules that are part of Rockwell’s equipment lineup and function under the Rockwell PLC ControlLogix system. This system oversees the entire process and ensures seamless integration with other modules in the process.
The biologists do not need to design the equipment or program it. They just have to communicate their needs to Rockwell support personnel – determining the necessary cell quantities, specifying durations for each step, or maintaining precise temperature and pH conditions – and they will articulate their requirements with reliability and documentation.

For Dean, the assurance provided by comprehensive documentation is crucial for proving not only the current production but also ensuring the ongoing capability:
“So I can prove to the FDA what I think I made and what I will continue to make.”
Although the foundry line we saw is a compact 20-foot-long system that produces bone ligaments every month, this is just the beginning. Dean expects a more disposable, potentially desktop-sized unit once production versions are ready.
“You’ll put your original cells in, and some number of days or weeks later, the product will come out.”
Rockwell also brings augmented reality capabilities. The technology allows users to visualize process data and equipment profiles in real-time, explained John Hatzis, technical consultant for Life Sciences with Rockwell Automation.
“This offers a comprehensive view of the manufacturing process, of the process data overlaying equipment, for example a reactor. We can demonstrate it on an iPad, but it is possible to integrate this into wearable technology. This can enable you to virtually view an unopened electrical cabinet, which is particularly significant in a clean room setting like a tissue foundry.”
What Has Been Produced So Far?
Even if biomanufacturing is fascinating, producing biological components on a large scale is undoubtedly challenging. For the moment, the 200 members of ARMI are working on prototypes and samples. Some members are dedicated to projects involving the creation of kidneys, lungs, individual blood cells, and even human hearts like Organamet Bio. Another member, Advanced Solutions, has designed a 3D printer equipped with a robotic arm that produces human tissue. But for the moment, we are far from being able to produce complete and functional human organs.
According to Tom, while progress is being made, the biology involved in organ creation still needs to catch up with the current technological capabilities.
“Beyond the sheer number of cells, the primary challenge lies in configuring human cells into a functional 3D structure mimicking an organ. This involves addressing issues related to nutrient and oxygen transport throughout the organ and the removal of waste materials and carbon dioxide.”
Having human organs might not be for tomorrow yet. But when the technology and manufacturing process are available, Dean says people will know where to come:
“Where do you go to find the skill sets, the people, the customers, to make organs? There isn’t a place yet. It’s going to be here.”









![Image [Best of 2025] Power Moves in the Energy World](/wp-content/uploads/sites/3/energy-320x213.jpg)