Paralysis caused by spinal cord injuries (SCI) has long been viewed as a permanent and devastating condition. But Netherlands-based medtech company Onward Medical is changing that narrative with ARC-EX, the first FDA-approved non-invasive spinal cord stimulation device designed to restore critical function to people living with SCI. Launched in the U.S. in December, ARC-EX is already showing life-changing results, offering new independence to patients — and renewed hope to their families and caregivers. In an exclusive interview, CEO Dave Marver shares insights on this breakthrough therapy.
When someone suffers paralysis from a severe spinal cord injury (SCI) even the smallest improvement in critical function can transform their lives and those of their families. Netherlands-based, med tech operation Onward Medical is already seeing ground-breaking results in the field with its innovative ARC-EX device, explains CEO Dave Marver.
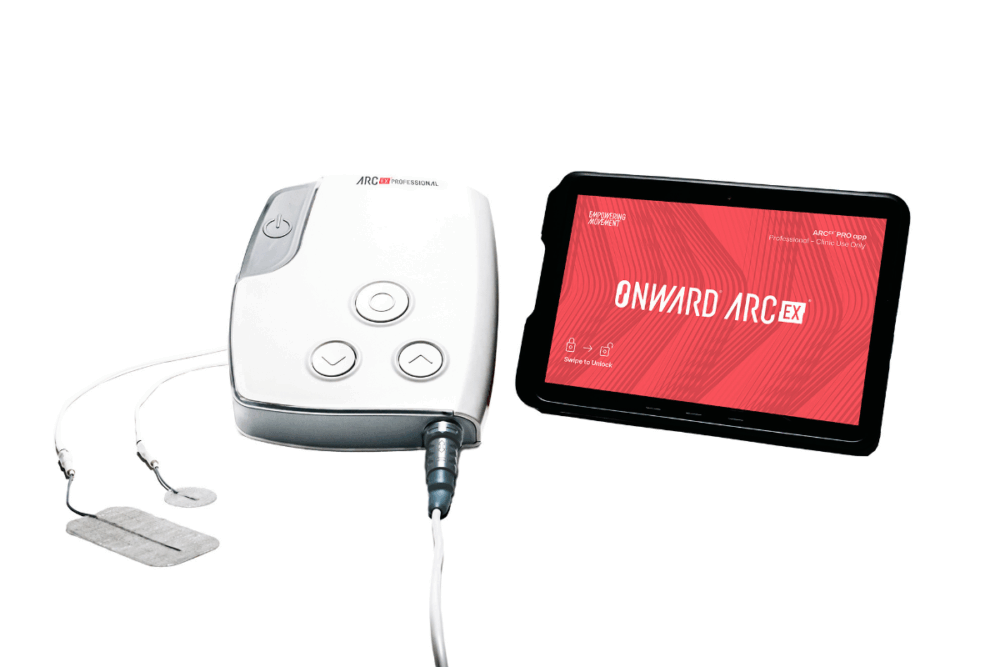
The first FDA-approved device for non-invasive spinal cord stimulation for people with SCI forms an intrinsic part of the pioneering company’s suite of ARC Therapy options. An external system, developed to offer non-invasive therapy, it has only been available in the US since December, with Europe and the UK expected to follow suit later this year.
“We informed the market we wanted to start with no more than 10 clinics and learn how to best introduce it for the long-term success of the therapy but there are already starting to be news reports about its success,” Mr. Marver explains. The human impact is extraordinary – any independence that we can help to introduce has a profound impact not only on the lives of those injured but also their loved ones and caretakers.”
You can find more details on this product in our sister publication MedicalExpo e-magazine.
A Suite of Technologies
Founded in 2014 by neuroscience researchers at the Swiss Federal Institute of Technology, Onward Medical is also developing implantable neuromodulation platforms for the delivery of its ARC Therapy. The ARC-IM implant has now been used in some 30 people in clinical feasibility studies and is expected to result in FDA and CE approval and CE mark within two to three years. A brain computer interface – with the aim of restoring thought-driven spinal stimulation and movement – is also in development.
“We want to bring forward technologies that can impact people with paralysis as quickly as possible,” Mr. Marver emphasizes. An external device has a less burdensome regulatory pathway. There’s no implant procedure so people don’t have to go into surgery to benefit from it. It is easier to administer the therapy. Having said that, it doesn’t have the benefits of an implantable. It’s not available 24/7 – it’s not a neuroprosthetic that can replace the brain signals on command like an implantable could. We think the external device is probably more applicable to people with less severe injuries, who don’t want a permanent implant, but maybe it is a bridge to the implant as well.”
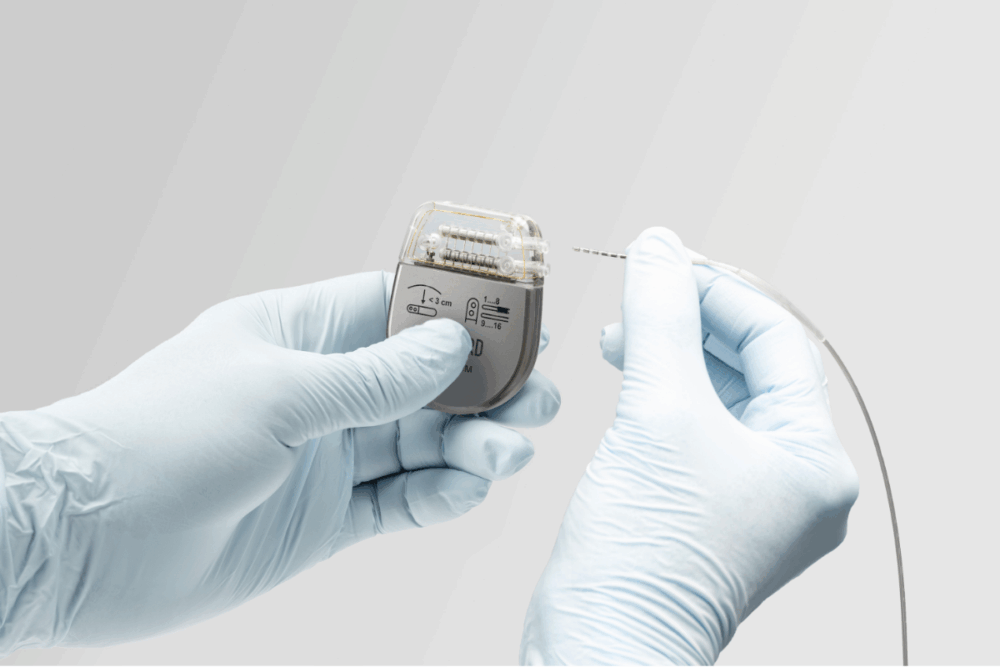

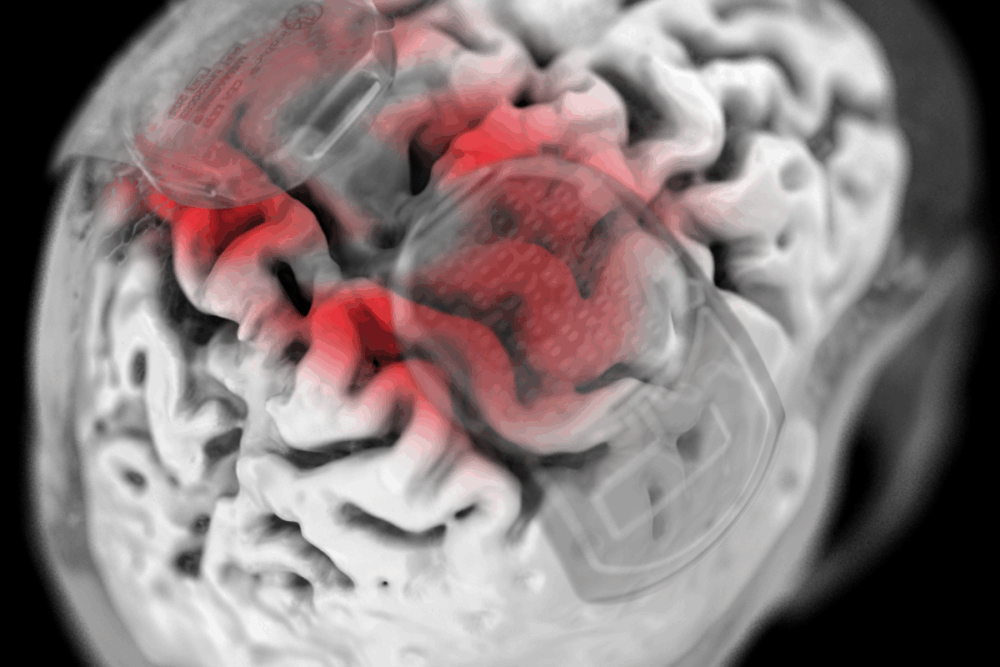
Rehabilitation After Injury
ARC-Ex has been approved for improvement of hand strength and sensation in people with spinal cord injury. Employed in a rehabilitation setting in combination with therapy, its pivotal study Up-LIFT focused on 65 people at 14 leading SCI centers in the US, Europe, and Canada and revealed improvements in participants up to 34 years post injury. The results also showed 90% of participants improved strength or function of their upper limbs and 87% reported improvement in quality of life.
“The tangible everyday impacts were that they recovered enough function to lift a filled cup or a fork, grasp a zipper or press a button on a remote control. These may seem like small things but they bring independence and reduce the need for outside assistance. Especially with improvements in hand function because we use it so much – even a 10% improvement makes a huge difference.”
Recovering Function
Electrodes are placed on the skin at the back of the neck to enable a stimulator to deliver electrical pulses to the cervical spinal cord.
“We administer the therapy and it excites the spinal cord in the region of injury and therefore elevates activity,” he explains. This allows a person to move in a way they otherwise could not and because they can move when it’s on, they can train when it’s on – and by training they can recover function.”
The device is priced at $39,500 in the US and the hope is that investment by clinics will significantly offset costs for ongoing outside care.
“We are also hopeful it will be available for use in the home by year-end,” says Mr. Marver. For us, this is a really important pursuit. If you have tetraplegia it is not always easy to get to the clinic so we want to make this available in the home where it can be used conveniently.”
Innovative and Impactful
Joining Onward Medical five years ago, Mr. Marver admits he takes every opportunity to interact with those who participate in the company’s studies.
“I see how challenging their lives can be and I see what kind of impact these therapies can have on them and I find it incredibly motivating. Every day counts – if we can spare them those challenges and introduce that function even one day sooner it can make a huge difference.”
He hopes to bring these promising therapies forward to the market in clinics all over the world, “as quickly as we can.”
Read also





![Image [BUYING GUIDE] How to Choose the Right Industrial Robot?](/wp-content/uploads/sites/3/Industrial-Robot-320x213.jpg)

![Image [Buying Guide] How to Choose the Right Safety Shoes?](/wp-content/uploads/sites/3/Safety-Shoes-320x213.jpg)


![Image [Buying Guide] How to Choose the Right AMR?](/wp-content/uploads/sites/3/AMR-320x213.jpg)